Authors: Pavan Undamattla, Surender Saini, and Pragya Pandey
Snowflake Native App Framework
Implementing a Clinical Trial Analytics system to streamline the entire clinical data analytics process. This framework provides sophisticated tools and functionalities to integrate multiple data sources like subject records, trial results, and regulatory data along with ensuring compliance with regulatory requirements by generating accurate and timely reports.
Enhancing Clinical Trials with Advanced Analytics
A clinical trial analytics system is designed to enhance the efficiency and effectiveness of clinical trials through data-driven insights and automation. It could optimize patient recruitment and enrollment, ensure real-time monitoring and compliance, improve operational efficiency and cost management, and provide comprehensive outcome analysis and reporting. By leveraging advanced analytics, the system can help streamline clinical trial processes, maintain regulatory compliance, reduce costs, and ultimately accelerate the development of new medical treatments.
Data Flow
Our application initiates by effortlessly fetching data stored in cloud storage. Leveraging the cloud’s reliability and scalability, users can securely access their data from anywhere, ensuring flexibility and convenience.
Upon data retrieval, the application automatically creates tables that match the data schema. This step eliminates the need for manual table setup, thus saving time and minimizing errors.
To enhance workflow efficiency, the application supports scheduled data processing tasks. Users can define specific time intervals or triggers for data retrieval, table creation, and data insertion, facilitating an automated and hands-free operation.
The application offers analytics capabilities, providing valuable insights into clinical studies and subjects. Users can generate reports, visualize trends, and gain a deeper understanding of their data, which supports informed decision-making and improves research outcomes.
Architectural Details
Implementation Details
- Go to the objects tab
Step 1: Creating Objects
The application is available on the Snowflake marketplace. Once installed, users need to go to the Objects tab. With a single click, users will be able to create all the essential objects such as database(CTAS), schema(OPENCLINICA ), and tables.
Step 2: Data Fetch
Once objects are created, users need to create an external stage. After the stage is set up, users will be able to fetch the data with a single click.
- Go to the Schedule tab
Step 3: Scheduling the stored procedures
Step 4: Visualizations for Clinical Trial Analytics System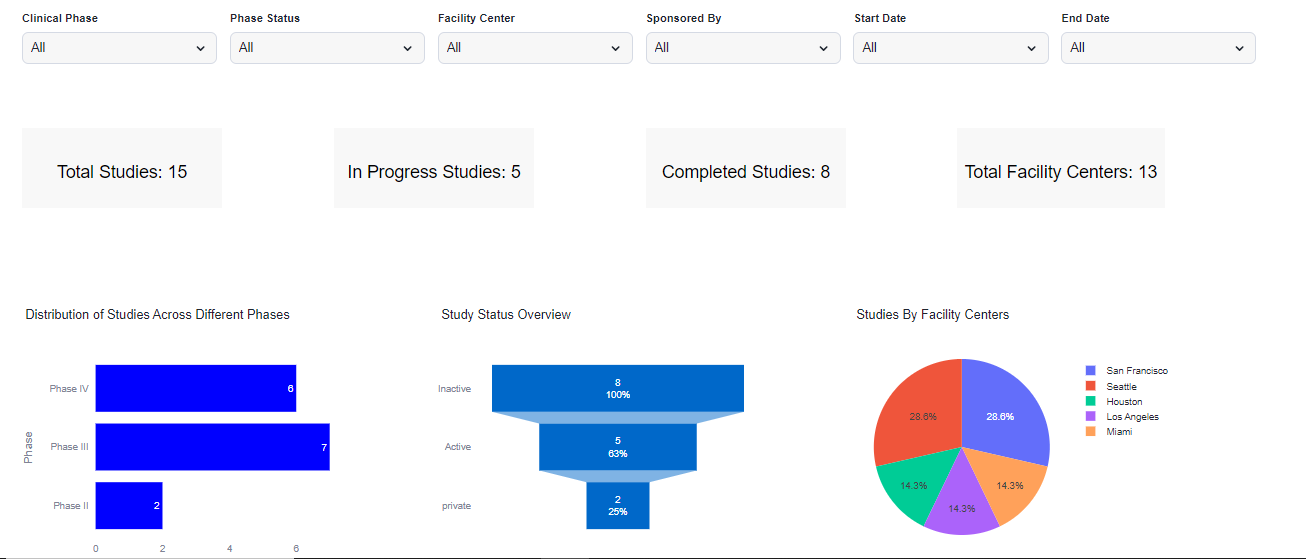
Use Cases and Benefits
Regulatory Compliance: Considering that the Clinical Trial space is heavily regulated due to its direct impact on patient lives, it becomes essential that all the regulatory compliances are addressed in a correct and timely manner. This not only ensures the timely completion of all filings that are required for Clinical Trial in the end but also prevents any penalty that may be associated with not following all the compliances, which again is a loss of revenue and prestige for Pharma companies. Thus a Clinical Trial Analytics system can help ensure compliance with regulatory requirements and streamline reporting to reduce overall administrative burden along with ensuring adherence to standards.
Regular Monitoring of Clinical Trials: Regular and real-time monitoring of clinical trial progress is crucial for ensuring the identification of risks to the clinical trial process and mitigation of the same. Monitoring of patient enrolments, adverse events, and other clinical trial progress indicators can help researchers make timely decisions and adjustments to the overall Clinical Trial process to achieve favorable outcomes.
Clinical Trial Optimization: The Clinical Trial Analytics system optimizes trial budgeting and resource allocation by providing comprehensive analytics on trial operations. It helps researchers identify inefficiencies, optimize site selection, and track financial metrics in real time, enabling data-driven decisions to reduce costs and improve trial efficiency.
Conclusion:
This comprehensive solution for clinical data management offers seamless cloud data retrieval, automated table creation, advanced data insights, and scheduled data processing. By leveraging the reliability and scalability of the cloud, users can access their data securely from cloud storage platforms, facilitating flexibility and convenience. With advanced analytics capabilities and automation features, researchers, clinicians, and healthcare professionals can make informed decisions, improve research outcomes, and optimize workflow efficiency. Overall, this streamlined approach to clinical data management empowers users to unlock the full potential of their data.







